Infection to Correction
You Covered
Infection to Correction
InterSpace® Knee ATS Spacer
With the same technology and proven science,1 we now offer a uniquely designed augmented tibial stem (ATS) that complements our existing knee spacer. This new component comes in multiple size and thickness options to best fit patient anatomy without sacrificing the stem length.

*7mm and 12mm thickness options
InterSep® Calcium Sulfate
Continuing our goal to provide infection-related solutions, Exactech introduces InterSep – a 100% synthetic calcium sulfate bone void filler engineered to fully resorb and replace bone during the healing process.
InterSep may be used in an infected bone site and provides surgeon flexibility with its bead and paste options.
Trust the Science.
High Release Matrix
Stainless Steel Core
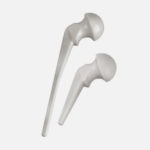
The stainless steel core, found in both the hip and shoulder spacers, provides a more robust mechanical structure.
Gentamicin Spectrum of Coverage
to non-infected revisions9
Eradication rate at latest follow-up10
From Primary to Revision – Comprehensive and Versatile
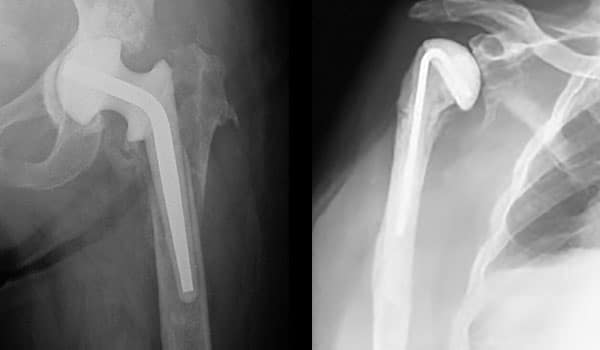
When cases call for revisions, we are here from primary to revision and infection to correction.
And it doesn’t stop there. Contact Exactech to learn more about our full product scope.
1. Coffey MJ, et al. Treatment of glenohumeral sepsis with a commercially produced antibiotic-impregnated cement spacer. J Shoulder Elbow Surg. 2010 Sep;19(6):868-73.
2. Magnan B, et al. Two-stage revision of infected total hip replacement using a pre-formed, \\antibiotic-loaded acrylic cement spacer. In: Meani E, et al [ed]. Infection and local treatment in orthopedic surgery. Berlin: Springer-Verlag; 2007. 205-13.
3. Diefenbeck, M, et al. Prophylaxis and treatment of implant-related infections by local application of antibiotics. Injury, 37(2). doi:10.1016/j.injury.2006.04.015.
4. Vecchini E, et al. Antibiotic-Loaded Spacer for Two-Stage Revision of Infected Total Knee Arthroplasty. J Knee Surg. 2017 Mar;30(3):231-237.
5. Romanò CL, et al. Preformed antibiotic-loaded cement spacers for two-stage revision of infected total hip arthroplasty. Long-term results. Hip Int. 2012 Jul-Aug;22 Suppl 8:S46-53.
6. Castelli CC, et al. Two-stage treatment of infected total knee arthroplasty: two to thirteen year experience using an articulating preformed spacer. Int Orthop. 2014 Feb;38(2):405-12.
7. Mutimer J, et al. Measurements of in vivo intra-articular gentamicin levels from antibiotic loaded articulating spacers in revision total knee replacement. Knee. 2009 Jan;16(1):39-41.
8. Soffiatti R. The Preformed Spacers: From the idea to the realization of an industrial device. In: Meani E, et al [ed]. Infection and local treatment in Orthopedic surgery. Berlin: Springer-Verlag. 2007;201-4.
9. Romanò CL, et al. Septic versus aseptic hip revision: how different? J Orthop Traumatol. 2010;11(3):167-174. doi:10.1007/s10195-010-0106-y.
10. Data on file at Exactech, Inc.
*Partial weight bearing must be assessed on an individual basis with relation to the anatomic condition of the local bone, bone quality and clinical conditions of the patient during rehabilitation stages. Care must be taken to minimize the risk of damaging bone tissue and the implant through excessive weight bearing or forced mobilization